
Читайте также:
|


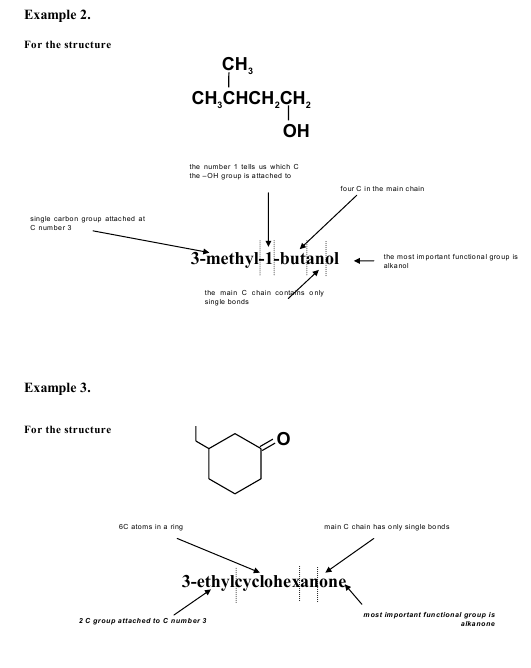
ISWT 8. Homologous series of oxygen-, nitrogen- and halogen- containing organic compounds. Carboxylic acids. Esters and ethers.
| Cn | Alkanoles (alcohols) | Alkanals (aldehydes) | Alkanones (ketones) | Amine (primary) |
| C1 | Methyl alcohol (methanol) | Methanal (formaldehyde) | - | Methylamine |
| C2 | Ethyl alcohol (ethanol) | Ethanal (acetaldehyde) | - | Ethylamine |
| C3 | Propyl alcohol (propanol) | Propanal (propionaldehyde) | Propanon (acetone) | Propilamine |
| C4 | Butyl alcohol (butanol) | Butanal (butyraldehyde) | Butanon | Butylamine |
| C5 | Pentyl alcohol (pentanol) | Pentanal (valeraldehyde) | Pentanon | Pentilamine |
| C6 | Hexyl alcohol (hexanol) | Hexanal (caproaldehyde, hexanaldehyde) | Hexanon | Hexylamine |
| C7 | Heptyl alcohol (heptanol) | Heptanal (heptaldehyde, enanthic aldehyde) | Heptanon | Heptylamine |
| C8 | Octyl alcohol (octanol) | Octanal (octaldehyde, caprylic aldehyde) | Octanon | Octylamine |
| C9 | Nonyl alcohol (nonanol) | Nonanal (nonaldehyde, pelargonic aldehyde) | Nonanon | Nonylamine |
| C10 | Decyl alcohol (decanol) | Decanal (decaldehyde, capric aldehyde) | Decanon | Decylamine |
| Polyhydric alcohols | ||
| C2H4(OH)2 | Ethane-1,2-diol | Ethylene glycol |
| C3H6(OH)2 | Propane-1,2-diol | Propylene glycol |
| C3H5(OH)3 | Propane-1,2,3-triol | Glycerol |
| C4H6(OH)4 | Butane-1,2,3,4-tetraol | Erythritol, Threitol |
| C5H7(OH)5 | Pentane-1,2,3,4,5-pentol | Xylitol |
| C6H8(OH)6 | Hexane-1,2,3,4,5,6-hexol | Mannitol, Sorbitol |
| C7H9(OH)7 | Heptane-1,2,3,4,5,6,7-heptol | Volemitol |
| Unsaturated aliphatic alcohols | ||
| C3H5OH | Prop-2-ene-1-ol | Allyl alcohol |
| C10H17OH | 3,7-Dimethylocta-2,6-dien-1-ol | Geraniol |
| C3H3OH | Prop-2-in-1-ol | Propargyl alcohol |
| Alicyclic alcohols | ||
| C6H6(OH)6 | Cyclohexane-1,2,3,4,5,6-hexol | Inositol |
| C10H19OH | 2 - (2-propyl)-5-methyl-cyclohexane-1-ol | Menthol |
Дата добавления: 2015-09-11; просмотров: 99 | Поможем написать вашу работу | Нарушение авторских прав |