
Читайте также:
|
Theme: molecular shape. The VSEPR Models of molecules.
Molecular shape is defined by the locations of a molecule’s atoms, not from the location of its electrons, because, experimentally, the scientist can determine the location of the atoms by X-ray diffraction and other studies. We can predict the shapes of molecules from their formulas by using a few simple rules. The ability to make such predictions enables us to understand better the properties of the substances.
To predict the shape of a molecule from its formula, we first draw an electron dot diagram of the molecule. We assume that the electron groups around the central atom are as far apart as possible while remaining attached to the central atom. The electron groups are ordinarily the electrons in the single, double, or triple bonds, or in the lone pairs on the central atom, which is the atom to which the other atoms are attached. Then we bond the other atoms, using some or all of the electron groups and finally describe the molecule by the locations of the atoms.
As a first example of the process, we consider the molecule BeH2 experimentally known to be a linear molecule with two identical bonds. The electron dot diagram has only four electrons around the beryllium, two from the outermost shell of that atom and one each from the two hydrogen atoms:
·Be·
We place the electron pairs as far apart as possible (180°) while still being attached to the Be atom. Then we add the two hydrogen atoms to form the molecule:
H·Be·H
We have deduced that the molecule is linear because all three atoms lie on a straight line.
Next, we consider BF3. The B atom has three outermost electrons to contribute. The three fluorine atoms each contribute their 1 unpaired electron, which makes a total of 6 electrons available. These are distributed in three pairs symmetrically about the B atom:

We add the three fluorine atoms and again get a symmetrical molecule:

In this case, we call the molecular shape trigonal planar because the triangle of fluorine atoms and the boron atom all lie in the same plane. Our next example is methane. The 4 outermost carbon electrons plus the 4 electrons from the hydrogen atoms total 8. These are distributed in four pairs as far apart as possible. In this case, the distribution is toward the corners of a tetrahedron.
Note that the electrons are not limited to a single plane. The addition of the hydrogen atoms produces a tetrahedral molecule.
Ammonia, NH3 is our next case. The 5 outermost electrons in the nitrogen atom, plus the 3 electrons from the three hydrogen atoms, again make 8. Once more, these are distributed toward the corners of a tetrahedron:

This time, however, only three hydrogen atoms are to be attached. Although the electrons are located toward the corners of a tetrahedron, the molecular shape is called trigonal pyramidal, not tetrahedral, because the atoms lie at the corners of a triangular pyramid:

In summary, the four electron pairs tetahedrally oriented around a central atom give rise to different molecular geometries because of the different numbers of atoms that are attached.
Compounds with multiple bonds are only slightly different. The electrons in each multiple bond are considered to be one group of electrons.

Molecular Structure: The VSEPR Model
The structures of molecules play a very important role in determining their chemical properties. As we will see later, this is particularly important for biological molecules; a slight change in the structure of a large biomolecule can completely destroy its usefulness to a cell or may even change the cell from a normal one to a cancerous one.
Many accurate methods now exist for determining molecular structure, the three-dimensional arrangement of the atoms in a molecule. These methods must be used if precise information about structure is required. However, it is often useful to be able to predict the approximate molecular structure of a molecule. In this section we consider a simple model that allows us to do this. This model, called the valence shell electron-pair repulsion (VSEPR) model, is useful in predicting the geometries of molecules formed from nonmetals. The main postulate of this model is that the structure around a given atom is determined principally by minimizing electron-pair repulsions. The idea here is
that the bonding and nonbonding pairs around a given atom will be positioned as far apart as possible. To see how this model works, we will first consider the molecule BeCl2, which has the Lewis structure

Note that there are two pairs of electrons around the beryllium atom. What arrangement of these electron pairs allows them to be as far apart as possible to minimize the repulsions? Clearly, the best arrangement places the pairs on opposite sides of the beryllium atom at 180 degrees from each other:

This is the maximum possible separation for two electron pairs. Once we have determined the optimal arrangement of the electron pairs around the central atom, we can specify the molecular structure of BeCl2, that is, the positions of the atoms. Since each electron pair on beryllium is shared with a chlorine atom, the molecule has a linear structure with a 180-degree bond angle:

Next, let’s consider BF3, which has the Lewis structure

Here the boron atom is surrounded by three pairs of electrons. What arrangement will minimize the repulsions? The electron pairs are farthest apart at angles of 120 degrees:

Since each of the electron pairs is shared with a fluorine atom, the molecular structure will be

This is a planar (flat) and triangular molecule, which is commonly described as a trigonal planar structure.
Next, let’s consider the methane molecule, which has the Lewis structure

There are four pairs of electrons around the central carbon atom. What arrangement of these electron pairs best minimizes the repulsions? First, let’s try a square planar arrangement:

The carbon atom and the electron pairs are centered in the plane of the paper, and the angles between the pairs are all 90 degrees.
Is there another arrangement with angles greater than 90 degrees that would put the electron pairs even farther away from each other? The answer is yes. The tetrahedral structure has angles of 109.5 degrees:
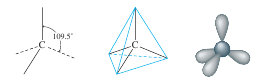
It can be shown that this is the maximum possible separation of four pairs around a given atom. This means that whenever four pairs of electrons are present around an atom, they should always be arranged tetrahedrally.
Now that we have the electron-pair arrangement that gives the least repulsion, we can determine the positions of the atoms and thus the molecular structure of CH4. In methane, each of the four electron pairs is shared between the carbon atom and a hydrogen atom.
The molecule has a tetrahedral structure with the carbon atom at the center.
Recall that the main idea of the VSEPR model is to find the arrangement of electron pairs around the central atom that minimizes the repulsions. Then we can determine the molecular structure from knowing how the electron pairs are shared with the peripheral atoms. Use the following steps to predict the structure of a molecule using the VSEPR model.
ISWT 6. Oxydation-reduction reactions (redox-reactions).
Reactions, in which one or more electrons are transferred, are called oxidation–reduction reactions or redox reactions.
We define the oxidation states (or oxidation numbers) of the atoms in a covalent compound as the imaginary charges the atoms would have if the shared electrons were divided equally between identical atoms bonded to each other or, for different atoms, were all assigned to the atom in each bond that has the greater attraction for electrons.
Oxidation–reduction reactions are characterized by a transfer of electrons. In some cases, the transfer occurs in a literal sense to form ions.
Дата добавления: 2015-09-11; просмотров: 105 | Поможем написать вашу работу | Нарушение авторских прав |