
Читайте также:
|
Crude oil is mixture of many different hydrocarbons - chemicals which contain only hydrogen and carbon atoms.
Crude oil is not much use until it is separated into more useful parts, called fractions. This is done by fractional distillation.
Most of the fractions are burnt as fuels. The rest go to make plastics, detergents and many other important chemicals.
1. What is a hydrocarbon?
2. Octane is a hydrocarbon which has eight carbon atoms. Which fraction would you find it in?
3. Which property of hydrocarbons is used to separate them?
4. Which fraction has the lowest boiling range?
5. Which fraction would be the hardest to boil?
6. Which of the fractions are burnt as fuels?
7. Which fraction do you think there is most demand for in the world?
8. What do you think would happen to the price of crude oil if:
(a) all countries banned the use of nuclear power?
(b) the Persian Gulf, through which most of the West's oil is carried, was
closed by war?
9. Decide what you think might happen if the oil runs out.
10. Write a paragraph of 100-150 words explaining what you think will happen.
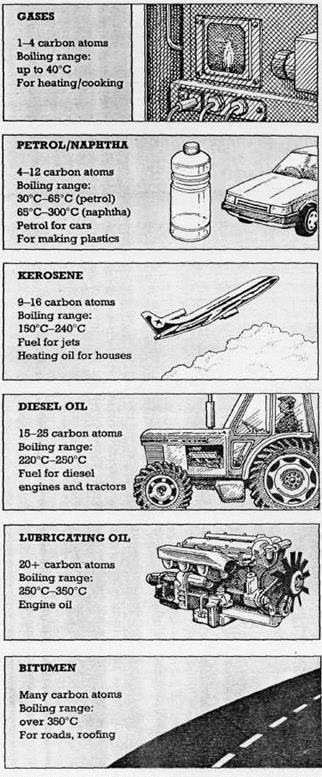
Fig.7. The Fractions
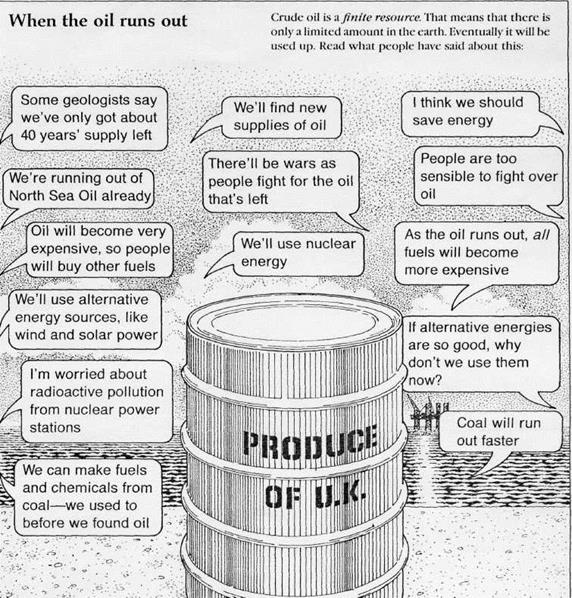
Fig.8.When the oil runs out.
(Stephen Beer, David Edwards “Thinking Through Science”, London, 1989).
Дата добавления: 2015-09-10; просмотров: 83 | Поможем написать вашу работу | Нарушение авторских прав |